The people from Ratnagiri district will be participating in screening camps arranged in each village, wherein they will be screened for ‘Double contrast Barium Swallow’.
Background
- Esophageal and Hypopharyngeal cancer are amongst the five leading cancers in India. In Dibrugarh Assam registry they both togather lead the list of cancers.
- Hypopharynx cancers are commoner in the developing countries as compared to the western region.
- There is no screening policy in place for these cancers and they are mostly diagnosed in advanced stages.
Project goals
Use of double contrast barium swallow as a screening tool to reduce cancer related mortality due to esophageal and hypopharyngeal cancers.
Project objectives
To Assess
- Improvement in survival after diagnosis of esophageal and hypopharyngeal cancers.
- Sensitivity and Specificity of double contrast barium swallow (with spot film) in diagnosis of asymptomatic esophageal and hypopharyngeal cancers.
- Cost-effectiveness of mass screening for the above said cancers in the community.
- Identification of possible etiological factors for the above said cancers.
Outcomes of our project
- Reduction in Esophageal and hypopharyngeal cancers related mortality.
- Improvement in the prognosis of the above said cancers.
- Effectiveness of DDCBS for diagnosis of the above said cancers.
- Affordability of the screening process for the above said cancers.
- To obtain associations between possible lifestyle and socio-demographic factors for causation of the above said cancers.
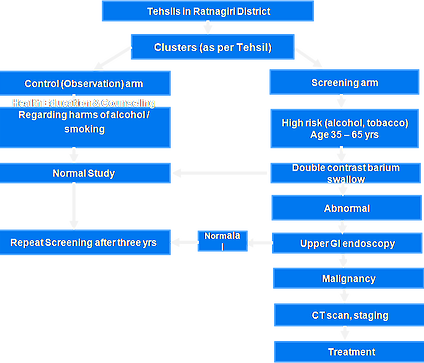
Project Design and Implementation
- Study Design: Prospective community based cluster randomized clinical trial
- Collaborating Institution: BKLW Hospital, Dervan
- Study Site: Ratnagiri District.
– Clusters will be formed and randomised into two arms.
– Base Hospital: BKLW Hospital, Dervan, Chiplun
Eligible subjects
- Healthy
- Ambulant men and women
- Tobacco and Alcohol users as risk factors
- Permanent residents in the chosen clusters.
Inclusion Criteria
- Subjects with risk factor exposure for esophageal and hypopharyngeal cancers-
- Chronic tobacco users (cumulative use of more than 5 years)
- Betel and areca nut chewers
- Alcohol use (cumulative more than 5 years)
- Age: 35-65 years.
Study Groups
Group 1: Control Arm
Group 2: Screening Arm
Sample Size: -55,000 subjects in each arm
-7500 subjects in each cluster (approximately).
-7 clusters in each arm
Control Arm
- This is an Observation arm.
- No active intervention is going to be provided to the subjects in this arm.
- Subjects selected for this arm will be similar to the subjects of the screening arm in all respects.
- The subjects of this arm will receive health education and counseling regarding the harmful effects of tobacco and alcohol.
Screening Arm
- The subjects of this arm will receive health education and counseling regarding the harmful effects of alcohol and tobacco.
- The subjects will also receive awareness sessions on cancer diagnosis and the follow up for positive cases emphasizing compliance to the study and requisite follow-up.
- The subjects of this arm will also receive an intervention.
Place of Screening
- In the selected community.
- MESU (Mobile Esophageal Screening Unit) with a digital radiography machine installed will be used for this study.
- Images obtained will be interpreted and reported by a qualified radiologist
Follow-up Schedule
- 2 rounds of screening
- 1st round-1st -3rd years
- 2nd round- 4th -6th Year
- Follow- up surveillance -4 years.
Documentation
- Household Survey Form
- Eligibles register
- Registration Registers
- History sheet
- Screening Form
- Follow-up registers
- Hospital Documentations
Study protocol, Logistics for the Hypopharyngeal and Esophagus cancer screening study.
Identifying Stake holders
- Listing of Stakeholders
- Contact programmes with Stake holders
- Project Information material for Stake holders
- Planning Awareness sessions for Stake holders
Health Education Program
- Health Education material for general population awareness programmes.
- Different mediums and tools for general population awareness programmes.
- Organisation of Health Education programmes (Day time & Night Time)
- Formation of Teams
Household Surveys for Eligible population
- Household Survey forms
- Eligible population listing: current and compatibility
- Check list for completeness of Survey
Screening Forms
- Screening forms data collection
- Defining Eligibility criteria
- Check list for completeness of Forms
Summary
- Of the total 34332 eligible, 20554(60%) attended DCBS screening.
- 37(0.2%) screen positive, 37(100%) attended follow up , 16 cancer cases detected (0.08). Out of 16 cancer cases, 15(93.8%) cases completed treatment.
- Follow up of DCBS screening from Ratangiri cluster is going on.
- Oral Cancer Screening is in process at Lanja, Mandangad and at Dapoli.
- Of the total 48402 eligible, 34467 (71.2%) attended oral cancer screening. 809 (2.3%) screen positive, 719(89%) attended follow up. 21(0.06%) cancer cases detected.
- Out of 21 oral cancer cases 17 (81%) cases has completed treatment.
- In the control arm, 22377 eligible recruited.
- The cancer cases data collection in intervention arm and control is in process.
- The mortality data collection is in process.